Electric vehicles are booming. But so is the problem of recycling the batteries inside them. EV battery packs are complex, made of tightly bound metals, flammable electrolytes, and components that are difficult to separate without heavy processing. A team at MIT has come up with a clever molecular design that could let part of a battery fall apart safely at end of life—making recycling simpler, cheaper, and more environmentally friendly.
What They Did
-
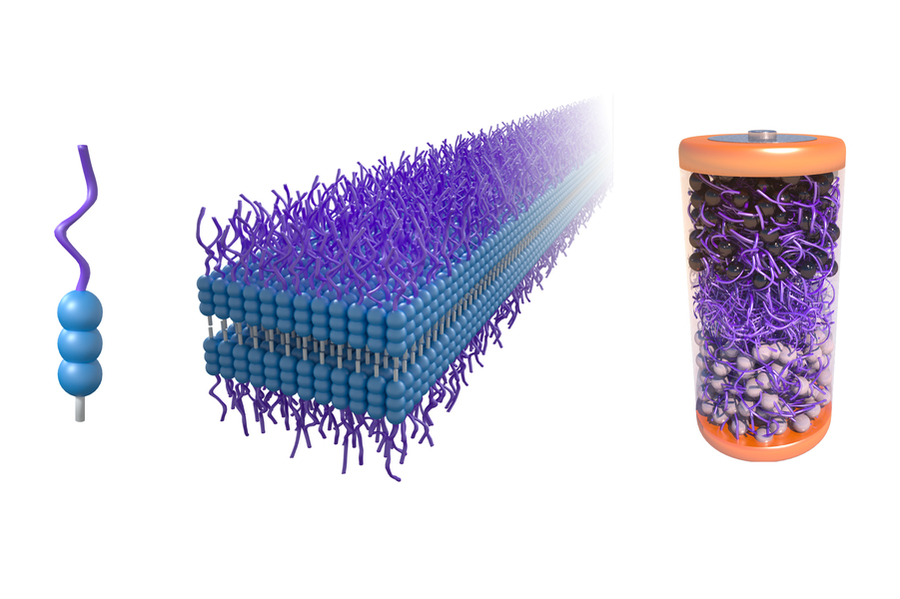
The researchers designed a new kind of electrolyte using molecules called aramid amphiphiles (AAs). These molecules are water-friendly on one end, and robust (think Kevlar-like strength) on the other.
-
In water, these molecules self-assemble into nanoribbons. The ribbons’ structure conducts lithium ions (via polyethylene glycol, or PEG, portions of the molecules), while maintaining mechanical stability via the Kevlar-like parts.
-
When dried and hot-pressed, these nanoribbons form a solid-state electrolyte layer. The team built test solid-state cells (with lithium iron phosphate cathodes, lithium titanium oxide anodes) to verify ionic conduction and mechanical robustness.
The Recycling Magic Trick
Here’s where it gets interesting: when the battery with this electrolyte is immersed in a common organic solvent (i.e. solutions readily available in recycling plants), the electrolyte dissolves or disperses, letting electrodes and other components detach cleanly. It’s like the glue holding things together vanishing—no need for brutal shredding, heat, or harmful chemicals.
Trade-Offs & Challenges
-
Performance isn’t yet on par with top commercial cells: while ion movement through the nanoribbon network works well, moving lithium ions from those ribbons into the solid electrodes (metal oxides) was slower, especially during fast charge/discharge sequences. That “interfacial polarization” is the current bottleneck.
-
The material in this study is a proof of concept. The researchers note that the new electrolyte might initially be used as part of a composite electrolyte rather than the entire thing, easing integration.
Why This Matters to Engineers & Makers
-
Design for disassembly: Building materials that naturally come apart can massively reduce the cost and energy footprint of recycling EV batteries.
-
Material innovation: Using self-assembling molecules means simpler fabrication (water actuation, self-assembly) and less harsh chemical processing later.
-
Sustainability & supply chain: As demand for lithium and other battery metals rises, being able to reuse components helps buffer supply shocks and reduce environmental footprint.
What’s Next
-
Optimize the interface between the new electrolyte ribbons and conventional electrode materials to reduce ion transfer resistance.
-
Test long-term cycling, durability under different conditions (temperature, temperature swings, mechanical stress).
-
Find ways to work this material into commercially relevant solid-state or hybrid battery designs.
-
Scale up: proving manufacturability, cost, and compatibility with existing battery assembly lines will be essential.
Original Story: New self-assembling material could be the key to recyclable EV batteries | MIT News | Massachusetts Institute of Technology