With lithium-ion batteries powering almost all remote devices, their manufacturers have had to develop safety measures to limit peak voltage. These include devices that monitor charge-state and current flow, record the latest, full-charge capacity, and monitor temperature. Designed to minimize the risk of short circuits, it’s not so much that they catch fire frequently as that there is always the possibility they might do so.
Current lithium-ion batteries safety issues can damage equipment too. Recently, researchers at the Graduate School of Engineering and Graduate School of Science at the University of Tokyo came up with a way to improve safety and provide more charge.
For the first time, researchers who explore the physical and chemical properties of electrical energy storage have found a new way to improve lithium-ion batteries. They successfully increased not only the voltage delivery of a lithium-ion battery but also its ability to suppress dangerous conditions that affect the current range of batteries. This improved lithium-ion battery could make longer journeys in electric vehicles possible and lead to the creation of a new generation of home energy storage, both with improved fire safety.
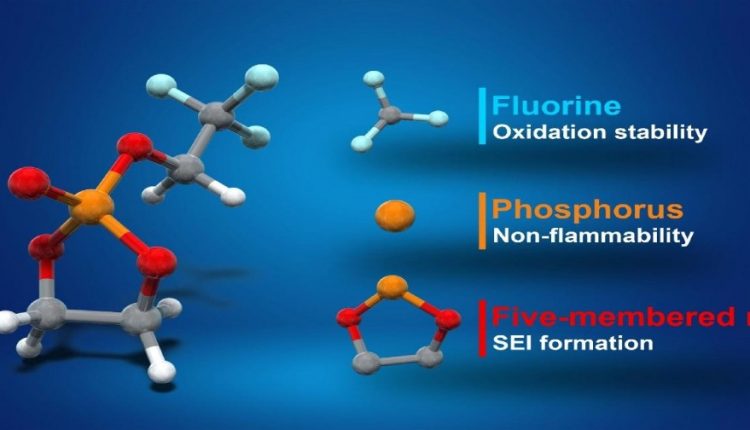
“A battery’s voltage is limited by its electrolyte material. The electrolyte solvent in lithium-ion batteries is the same now as it was when the batteries were commercialized in the early 1990s,” said Professor Atsuo Yamada. “We thought there was room for improvement, and we found it. Our new fluorinated cyclic phosphate solvent (TFEP) electrolyte greatly improves upon existing ethylene carbonate (EC), which is widely used in batteries today.”
EC is notoriously flammable and is unstable above 4.3 volts; TFEP, on the other hand, is nonflammable and can tolerate greater voltages of up to 4.9 volts. This extra voltage in an otherwise identically sized package can mean the batteries can last longer before they need another charge. As lithium ion-powered electric vehicles proliferate, this extra range and safety would no doubt prove extremely useful.
“We’re proud of this development and its effectiveness came as a bit of a surprise. This is because the way we came up with TFEP was novel in itself, thanks in part to our collaboration with organic chemist Professor Eiichi Nakamura,” continued Yamada. “Most research on electrolytes is a bit trial and error, with slight alterations to the basic chemistry rarely offering any advantage. Our approach came from a theoretical understanding of the underlying molecular structures. We predicted the safe, high-voltage properties before we experimentally verified them. So it was a very pleasant surprise indeed.”
Source: University of Tokyo
