Clean fuel from hydrogen energy source
Researchers have found a way to weaken the strong bonds between the nitrogen and hydrogen atoms in ammonia molecules while simultaneously releasing a single hydrogen atom to create hydrogen gas. In their paper published in the journal Science, Máté Bezdek, Sheng Guo and Paul Chirik describe the process and the likelihood of using it as a new hydrogen source. Jessica Hoover with West Virginia University offers a Perspective piece on the work done by the team in the same journal issue and also outlines the impact the findings are likely to have on hydrogen energy storage and utilization.
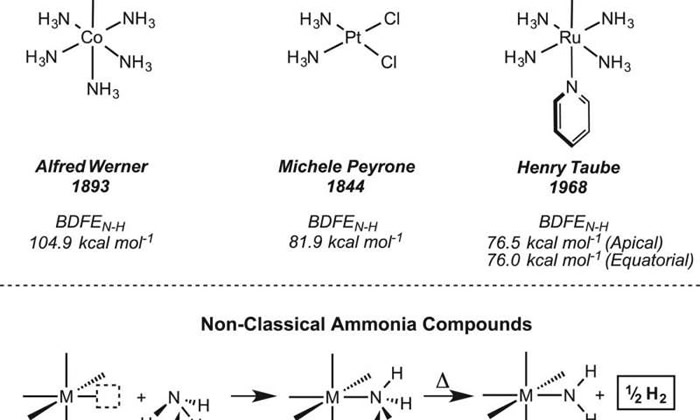
Ammonia has long been used on a large scale to produce both fertilizers and feedstock, and on a smaller scale as a household cleaner, but scientists would also like to use it as a type of fuel, because doing so would offer a new way to create hydrogen gas for use as a clean energy source. But to date, efforts to do so have been stymied by the strong bond that exists between the nitrogen and hydrogen atoms in ammonia molecules. In this new effort, the researchers report that they have found an efficient means for doing so that not only weakens the bond, but also causes the release of one of the hydrogen atoms making it available to bond with another to create hydrogen gas.
The new process involves using an ammonia-bound terpyridine bis(phosphine) molybdenum(I) cation, because as Hoover notes, it is both electron rich and positively charged. In so doing, the nitrogen–hydrogen bond is cleaved homolytically, resulting in a lone hydrogen atom and an M–N bond. The newly freed hydrogen atom is then able to bond with another to form hydrogen gas, which can then be collected, stored and eventually burned to provide energy for a variety of engines.
The work by the team, Hoover also notes, is likely to have an impact both on energy systems (the process is much more efficient than other methods such as deprotonation or oxidative addition) and on other work that involves synthesizing ammonia, perhaps leading to even more efficient processes.
More information: Phys.org

Comments are closed, but trackbacks and pingbacks are open.