More than 8 billion people are alive on Earth today. The more our population increases, the more strain we put on our resources. Scientists are looking at insect protein as an integral part of feeding our future world. In fact, the insect farming market is expected to reach $7.96 billion by 2030.
I’ve tasted mealworms and crickets. They’ve got an underlying nutty flavor and are all right… as long as you can keep the cricket legs from sticking in your teeth.
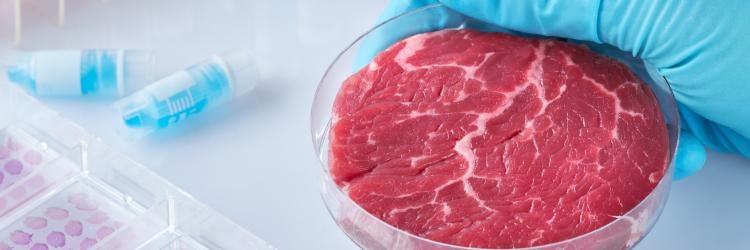
Scientists are also looking at cellular agriculture—a process that grows meat in bioreactors. This tech has several challenges to overcome in order to be viable to feed billions. One of the main challenges is the fact that muscle and fat cells drawn from live animals typically divide about 50 times before they are “old,” and no longer viable.
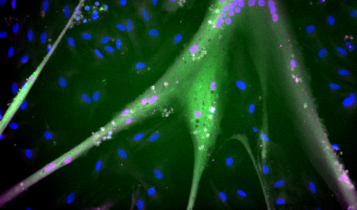
Researchers at Tufts University Center for Cellular Agriculture (TUCCA) are developing immortalized bovine muscle stem cells (iBSCs) that can grow rapidly and divide hundreds of times. Possibly indefinitely. They published their research in Synthetic Biology so that researchers and countries worldwide can develop new products without having to source cells repeatedly.
Current cell-cultured meat products are expensive and difficult to scale. (Remember the Wooly Mammoth Meatball? And the recently FDA-approved “cultured chicken” by Upside Foods?) The immortalized cells developed by the TUCCA team may solve many of these issues. They can create significantly more meat and lower the barrier of entry for other companies to explore cellular agriculture.
Most cells begin to lose DNA cells at the ends of their chromosomes as they age. This loss can lead to errors as the DNA copies and repairs itself, causing gene loss and, eventually, death.
The researchers engineered the bovine stem cells to resist this DNA fraying by constantly rebuilding their telomeres (the proteins at the end of DNA), setting the cells up for more rounds of replication and cell division. They also caused the cells to continuously produce a protein that stimulates a critical stage of cell division, “supercharging” the entire process and causing cells to grow faster.
While there are still issues the team must address (the muscle stem cells must differentiate into “mature muscle cells” like those we currently eat), the team hopes that others will join in the efforts to explore the possibilities of cell-cultured proteins more fully.