First-of-their-kind snapshots reveal byproduct crippling powerful, experimental cells
It’s been long known that lithium-metal batteries hold 50% more energy than their lithium-ion counterparts, but they are prone to failure and such safety problems as fires and explosions. The why behind that has been elusive.
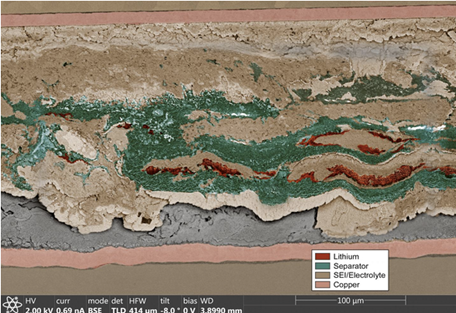
Now, the first images of the inside of the batteries tell the story. The culprit? Internal byproduct builds up, kills batteries and a hard buildup formed as a byproduct of the battery’s internal chemical reactions. When recharged, the byproduct, called solid electrolyte interphase, grew. Capping the lithium shredded the separator, creating openings for metal deposits to spread and form a short.
Sandia scientists, in collaboration with Thermo Fisher Scientific Inc., the University of Oregon and Lawrence Berkeley National Laboratory, published the images in ACS Energy Letters.
Determining cause-of-death for a coin battery is surprisingly difficult because of its stainless steel casing which prevents imaging from the outside, while removing parts of the cell for analysis rips apart the battery’s layers and distorts whatever evidence might be inside.
The original demonstration instrument, which was the only such tool in the United States at the time, was built and still resides at a Thermo Fisher Scientific laboratory in Oregon. An updated duplicate now resides at Sandia. The tool will be used broadly across Sandia to help solve many materials and failure-analysis problems.
Original Release: Eureka Alert
