
Researchers develop self-heating Lithium-ion battery for all weather conditions
A common problem with conventional batteries is that they lose power when they encounter below-freezing temperatures, resulting in slower charging times. Dealing with these issues typically requires larger and expensive battery packs to compensate.
But now, a team of researchers from Penn State and EC Power, State College have developed a lithium-ion battery that self heats if the temperature drops below 32° F. While the new battery has multiple applications, the researchers are exploring its potential impact on winter “range anxiety” for electric vehicle owners.
“It is a long standing problem that batteries do not perform well at subzero temperatures,” said Chao-Yang Wang, William E. Diefenderfer Chair of mechanical engineering, and professor of electrical and chemical engineering. “This may not be an issue for phones and laptops, but is a huge barrier for electric vehicles, drones, outdoor robots and space applications.”
Recently, the American Automobile Association reported that electric cars lose 40% to 50% of their cruise range when the temperature drops, which inspired the team’s new battery.
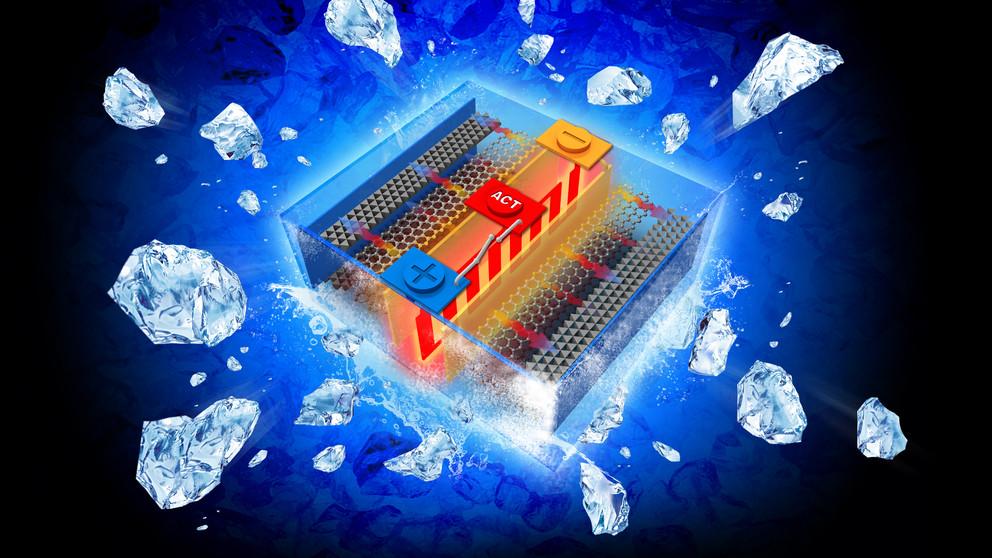
The researchers relied on previous patents by EC Power to develop the all-climate battery that weights just about 1.5% more and costs about 0.04% of the base battery. They designed it to go from -4° to 32° F within 20 seconds and from -22° to 32°F in 30 seconds, all while consuming only 3.8% and 5.5% of the cell’s capacity. (Less than the 40% loss in conventional lithium ion batteries.)
The all-climate battery uses a nickel foil that is 50-micrometers thick and has one end attached to the negative terminal and the other extending outside the cell to create a third terminal. The team attached a temperature sensor to a switch that causes electrons to flow through the nickel foil to complete the circuit. This sensor causes the nickel foil to heat up through resistance and warms the inside of the battery. Once the battery is at 32°F, the switch turns off and the electric current flows in the normal manner.
The team could have used other materials as a resistance-heating element, but nickel is less expensive and they found it to work well.
“Next we would like to broaden the work to a new paradigm called SmartBattery,” said Wang. “We think we can use similar structures or principles to actively regulate the battery’s safety, performance and life.”

Comments are closed, but trackbacks and pingbacks are open.